Molecular Radiooncology
In the last decades science gained better knowledge of processes and mechanisms in cancer cells and therapies were developed according to these findings. Radiotherapy and chemotherapy are two of the most important strategies for the treatment of patients with cancerous malignancies. Improvement of systemic chemotherapy led to innovations such as targeted therapy and immunotherapy by counteracting cancer cells with antibodies and small molecule kinase inhibitor (smKI). Furthermore, modern adjuvant radiotherapy improves local control and reduces cancer-specific mortality. To go further in cancer therapy, the combination of several different therapies is becoming more and more crucial, especially in the context of personalized medicine. We want to have a closer look at prospective and promising combinations based on radiotherapy and drugs targeting cancer-related pathways and their potential to affect each other in different, highly relevant, entities such as breast cancer and head and neck cancer.

Dr. rer. nat. Tina Jost
Group lead
Phone: +49 9131 85-44276
E-mail: tina.jost(at)uk-erlangen.de

Anna Gottwald
PhD student
Phone: +49 9131 85-32311
E-mail: anna.gottwald(at)uk-erlangen.de
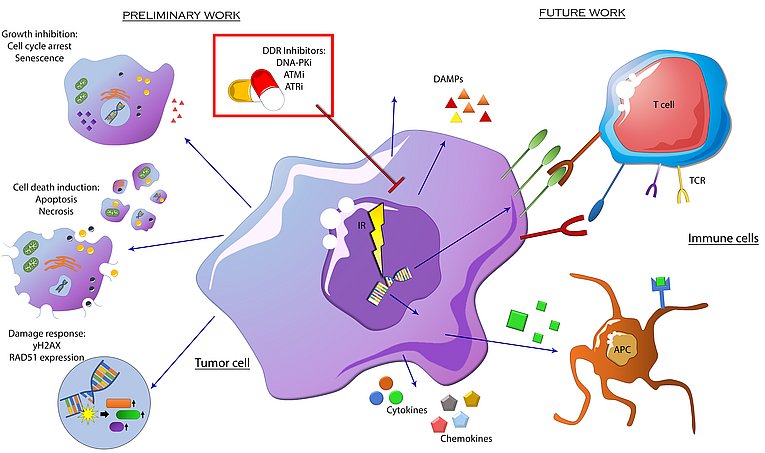
Optimizing the treatment of HNSCC by exploiting the radiosensitizing potential of targeted therapy.
Radiotherapy (RT) is a precise and locally applied treatment often used for tumors in the oral cavity or hypopharynx. It aims to cause DNA strand breaks (DSB) in tumor cells. The concomitant administration of drugs (called small molecule kinase inhibitors, smKI) to RT could help optimize the effectiveness of radiotherapy while reducing the risk related to normal tissue. This combination therapy could help reduce the risk of treatment failure in, for example, human papilloma virus (HPV)-negative head and neck squamous cell carcinoma (HNSCC) and also allow de-escalation of the applied dose to protect organs at risk in HNSCC. The research field of combining RT with kinase inhibitors continues to gain relevance in this regard, as many new targets in tumor cells (e.g., mutant proteins) have been identified in recent years and inhibitors that bind to them have been developed and approved. However, as there is still a lack of comprehensive data on the interaction of RT with most of the approved smKIs, we have investigated the impact of several inhibitors approved by the EMA or currently being tested in clinical trials in combination with RT and have already identified many additive and synergistic interaction effects.
Doctoral candidates - Medicine
Julia Meidenbauer (GRK2599)
Felix Deckwer
Anna Schäfer (IZKF funding)
Leonie Steinsdörfer (IZKF funding)
Selected Publications
Jost, T., Schuster, B., Heinzerling, L., Weissmann, T., Fietkau, R., Distel, L. V., & Hecht, M. (2022). Kinase inhibitors increase individual radiation sensitivity in normal cells of cancer patients. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al], 198(9), 838–848. https://doi.org/10.1007/s00066-022-01945-y
Scheper, J., Hildebrand, L. S., Faulhaber, E. M., Deloch, L., Gaipl, U. S., Symank, J., Fietkau, R., Distel, L. V., Hecht, M., & Jost, T. (2022). Tumor-specific radiosensitizing effect of the ATM inhibitor AZD0156 in melanoma cells with low toxicity to healthy fibroblasts. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al], 10.1007/s00066-022-02009-x. Advance online publication. https://doi.org/10.1007/s00066-022-02009-x
Jost, T., Schultz, A. K., Frey, B., Vu, J., Fietkau, R., Distel, L. V., & Hecht, M. (2022). Influence of alectinib and crizotinib on ionizing radiation - in vitro analysis of ALK/ROS1-wildtype lung tissue cells. Neoplasia (New York, N.Y.), 27, 100780. https://doi.org/10.1016/j.neo.2022.100780
Faulhaber, E. M., Jost, T., Symank, J., Scheper, J., Bürkel, F., Fietkau, R., Hecht, M., & Distel, L. V. (2021). Kinase Inhibitors of DNA-PK, ATM and ATR in Combination with Ionizing Radiation Can Increase Tumor Cell Death in HNSCC Cells While Sparing Normal Tissue Cells. Genes, 12(6), 925. https://doi.org/10.3390/genes12060925
Bürkel, F., Jost, T., Hecht, M., Heinzerling, L., Fietkau, R., & Distel, L. (2020). Dual mTOR/DNA-PK Inhibitor CC-115 Induces Cell Death in Melanoma Cells and Has Radiosensitizing Potential. International journal of molecular sciences, 21(23), 9321. https://doi.org/10.3390/ijms21239321
Further publications of Dr. Tina Jost available on "Web of Science".
Cooperation partner
Prof. Dr. Cornelia Brunner
Akademische Oberrätin, Wissenschaftliche Leitung des Forschungslabors, Klinik für Hals-Nasen-Ohrenheilkunde, Kopf- und Halschirurgie, Universitätsklinik Ulm, Deutschland