The current publication
Inhibitors of DNA repair: New approaches in combination with ionising radiation
Nina Klieber,Laura S Hildebrand, Eva Faulhaber, Julia Symank, Nicole Häck, Annamaria Härtl, Lukas Kuhlmann, Rainer Fietkau, Luitpold V Distel
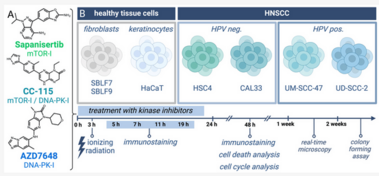
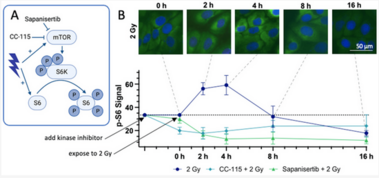
Despite substantial advancements in understanding the pathomechanisms of head and neck squamous cell carcinoma (HNSCC), effective therapy remains challenging. The application of kinase inhibitors (KIs) in HNSCC, specifically mTOR and DNA-PK inhibitors, can increase radiosensitivity and therefore presents a promising strategy when used simultaneously with ionizing radiation (IR) in cancer treatment. Our study focused on the selective DNA-PK-inhibitor AZD7648; the selective mTOR-inhibitor Sapanisertib; and CC-115, a dual inhibitor targeting both mTOR and DNA-PK. The impact of these KIs on HNSCC and normal tissue cells was assessed using various analytical methods including cell death studies, cell cycle analysis, real-time microscopy, colony-forming assays and immunohistochemical staining for γH2AX and downstream mTOR protein p-S6. We detected a strong inhibition of IR-induced DNA double-strand break (DSB) repair, particularly in AZD7648-treated HNSCC, whereas normal tissue cells repaired DNA DSB more efficiently. Additionally, AZD7648 + IR treatment showed a synergistic decline in cell proliferation and clonogenicity, along with an elevated G2/M arrest and cell death in the majority of HNSCC cell lines. CC-115 + IR treatment led to an elevation in G2/M arrest, increased cell death, and a synergistic reduction in cell proliferation, though the effect was notably lower compared to the AZD7648 + IR- treated group. Sapanisertib led to a high cellular toxicity in both HNSCC and normal tissue cells, even in non-irradiated cells. Regarding cell proliferation and the induction of apoptosis and necrosis, Sapanisertib + IR was beneficial only in HPV+ HNSCC. Overall, this study highlights the potential of AZD7648 as a radiosensitizing agent in advanced-stage HPV-positive and negative HNSCC, offering a promising therapeutic strategy. However, the dual mTOR/DNA-PK-I CC-115 did not provide a distinct advantage over the use of selective KIs in our investigations, suggesting limited benefits for its application in KI + IR therapy. Notably, the selective mTOR-inhibitor Sapanisertib was only beneficial in HPV+ HNSCC and should not be applied in HPV− cases.

The current publication
The inhibitor of apoptosis inhibition: xevinapant in head and neck tumours
Julia Fleischmann, Laura S Hildebrand, Lukas Kuhlmann, Rainer Fietkau, Luitpold V Distel
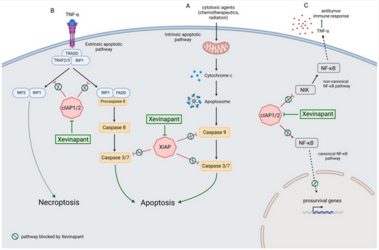
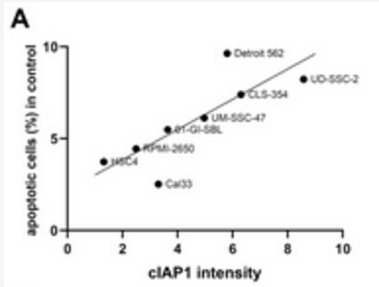
The poor prognosis of HNSCC is partly due to treatment resistance. The SMAC mimetic Xevinapant is a promising new approach to targeted cancer therapy. Xevinapant inhibits cIAP1/2 and XIAP, leading to apoptosis, necroptosis and inhibition of prosurvival signaling. Combining Xevinapant with IR could improve therapeutic potential. The effect of Xevinapant in combination with IR on HNSCC and healthy tissue cells was investigated. Cell growth, cell death, clonogenic survival and DNA double-strand breaks (DSBs) were studied, and intracellular cIAP1 and XIAP levels were evaluated. Xevinapant had cytostatic and cytotoxic, as well as radiosensitizing, effects on the malignant cells, while healthy tissue cells were less affected. Apoptotic and necrotic cell death was particularly affected, but the increase in residual DSBs and the reduced survival implied an additional effect of Xevinapant on DNA damage repair and other cell inactivation mechanisms. cIAP1 and XIAP levels varied for each cell line and were affected by Xevinapant and IR treatment. There was an association between higher IAP levels and increased cell death. Xevinapant appears to be a potent new drug for HNSCC therapy, especially in combination with IR. IAP levels could be an indicator for impaired DNA damage repair and increased susceptibility to cellular stress.

The current publication
Accelerated Partial Breast Irradiation: Can macrophage polarisation shift classification identify high-risk tumours in early breast cancer
Sören Schnellhardt, Ramona Erber, Maike Büttner-Herold, Marie-Charlotte Rosahl, Oliver J. Ott, Vratislav Strnad, Matthias W. Beckmann, Lillian King, Arndt Hartmann, Rainer Fietkau and Luitpold Distel
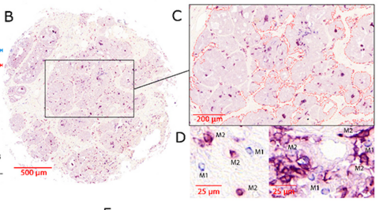
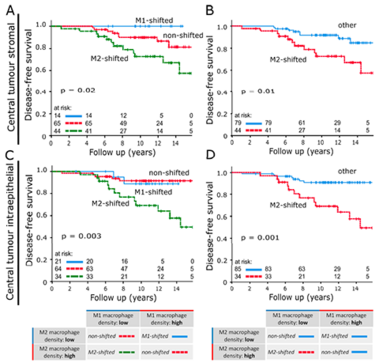
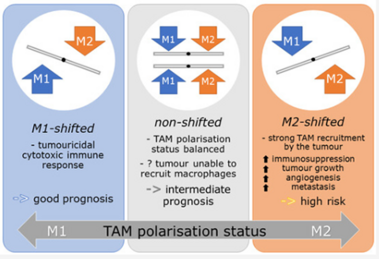
Studies have demonstrated correlations between accumulations of tumour-associated macrophages (TAMs), especially of M2-like phenotype, and increased mortality in advanced breast cancer. We investigated the prognostic potential of both main macrophage phenotypes in early hormone receptor-positive (HR+) breast cancer. The studied cohort of 136 patients participated in an institutional APBI phase II trial. Patient selection was characterized by HR+, small tumour size and no metastasis. Tissue microarrays from pre-RT resection samples were double stained for CD68/CD163 using immunohistochemistry. CD68+/CD163− cells were considered M1-like macrophages and CD68+/CD163+ was representative of M2-like macrophages. M1 and M2 macrophage densities were analysed semi-automatically in the stromal and intraepithelial tumour compartment. Low M1 and high M2 densities were strongly associated with decreased disease-free survival (DFS). Combined TAM phenotype densities were studied after defining a macrophage shift classification: M1-shifted (M1 high, M2 low) and non-shifted (M1 low, M2 low; M1 high, M2 high) tumours entailed a favourable outcome. In contrast, M2-shifted (M1 low, M2 high) TAM populations were associated with extremely reduced DFS. Thus, the full predictive potential of TAMs was revealed in a combined analysis of both phenotypes. The M2-shifted subgroup of tumours is classified as high-risk and probably not suitable for partial breast irradiation.

The current publication
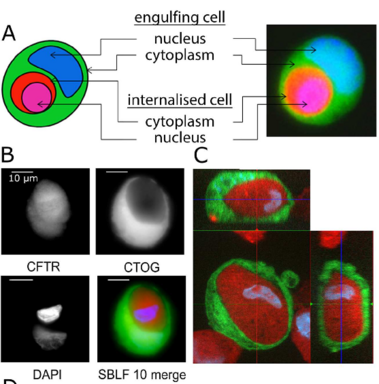
Increase in non-professional phagocytosis during the progression of cell cycle
[Translate to English:]
Increase in non-professional phagocytosis during the progression of cell cycle
Alexander Hofmann, Florian Putz, Maike Büttner-Herold, Markus Hecht Rainer Fietkau and Luitpold Distel
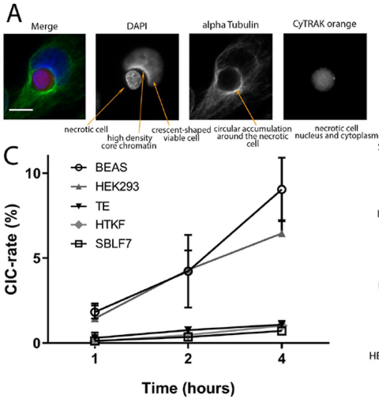
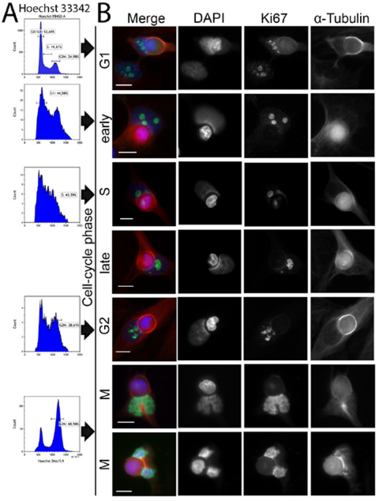
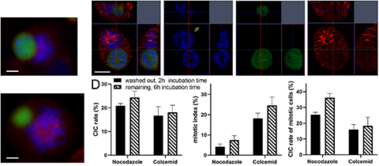
Homotypic or heterotypic internalization of another, either living or necrotic cell is currently in the center of research interest. The active invasion of a living cell called entosis and cannibalism of cells by rapidly proliferating cancers are prominent examples. Additionally, normal healthy tissue cells are capable of non-professional phagocytosis. This project studied the relationship between non-professional phagocytosis, individual proliferation and cell cycle progression. Three mesenchymal and two epithelial normal tissue cell lines were studied for homotypic non-professional phagocytosis. Homotypic dead cells were co-incubated with adherent growing living cell layers. Living cells were synchronized by mitotic shake-off as well as Aphidicolin-treatment and phagocytotic activity was analyzed by immunostaining. Cell cycle phases were evaluated by flow cytometry. Mesenchymal and epithelial normal tissue cells were capable of internalizing dead cells. Epithelial cells had much higher non-professional phagocytotic rates than mesenchymal cells. Cells throughout the entire cell cycle were able to phagocytose. The phagocytotic rate significantly increased with progressing cell cycle phases. Mitotic cells regularly phagocytosed dead cells, this was verified by Nocodazole and Colcemid treatment. Taken together, our findings indicate the ability of human tissue cells to phagocytose necrotic neighboring cells in confluent cell layers. The origin of the cell line influences the rate of cell-in-cell structure formation. The higher cell-in-cell structure rates during cell cycle progression might be influenced by cytoskeletal reorganization during this period or indicate an evolutionary anchorage of the process. Recycling of nutrients during cell growth might also be an explanation.
The current publication
Is radiation sensitivity measured on the basis of in vivo and ex vivo irradiation comparable?
Theresa Mayo , Marlen Haderlein, Barbara Schuster, Anna Wiesmüller, Christian Hummel, Maximilian Bachl, Manfred Schmidt, Rainer Fietkau and Luitpold Distel
Background: Individual radiosensitivity is influencing the outcome of radiation therapy. A general ex vivo testing
is very work-intensive. It is of interest to see if a significant prediction concerning the sensitivity can be made by
in vivo irradiation during radiation treatment.
Methods: Blood samples of 274 patients with rectal cancer and 43 lung cancer patients receiving radiotherapy
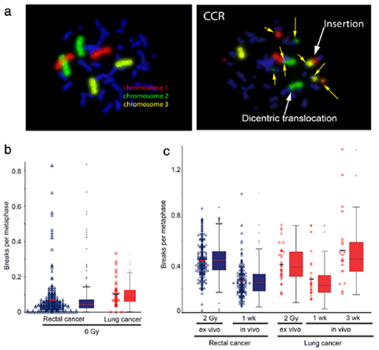
were examined after 2 Gy ex vivo and in vivo ionizing radiation. Chromosomes # 1, 2 and 4 were stained by the
3-color-fluorescence in situ hybridization. Chromosomal aberrations were analyzed as breaks per metaphase (B/M).
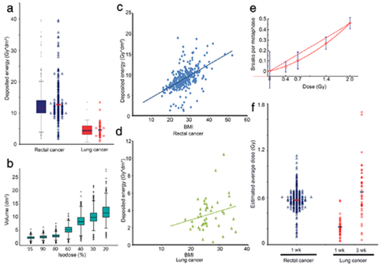
The deposited energy per session was calculated for each patient.
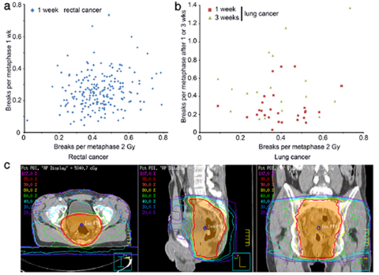
Results: Weak correlation could be found between the chromosomal aberrations ex and in vivo. Though receiving
significantly smaller deposited energy during radiation therapy (RT) the lung cancer cohort displayed B/M values
similar to the rectal cancer cohort. Considering the individual deposit energy differences improved slightly the
correlation.
Conclusions: As various factors influence the induction of chromosomal aberrations it seems not feasible to
estimate individual radiosensitivity via in vivo irradiation. An ex vivo estimation of individual radiosensitivity should
be preferred.
![[Translate to en:] Alessia Echarti CD8 FoxP3 Head and Neck Cancers](/fileadmin/_processed_/e/a/csm_Alessia_Header_f2b1552ae2.png)
![[Translate to en:] Alessia Echarti CD8 FoxP3 Head and Neck Cancers](/fileadmin/_processed_/b/0/csm_Alessia_Intro_0a8c296f1a.png)
![[Translate to en:] Alessia Echarti CD8 FoxP3 Head and Neck Cancers](/fileadmin/_processed_/8/d/csm_Alessia_DEI_89d59797cc.png)
![[Translate to en:] Alessia Echarti CD8 FoxP3 Head and Neck Cancers](/fileadmin/_processed_/8/c/csm_Alessia_GraphAbs_1d30165e56.png)
The current publication
"inflamed", "immune excluded" and "desert" with cytotoxic T cells and regulatory T cells
Alessia Echarti, Markus Hecht, Maike Büttner-Herold, Marlen Haderlein, Arndt Hartmann, Rainer Fietkau and Luitpold Distel
Background: The tumor immune status “inflamed”, “immune excluded”, and “desert” might serve as a predictive parameter. We studied these three cancer immune phenotypes while using a simple immunohistochemical algorithm. Methods: Pre-treatment tissue samples of 280 patients with locally advanced HNSCC treated with radiochemotherapy were analyzed. A double staining of CD8+ cytotoxic T cells (CTL) and FoxP3+ (Treg) was performed and the cell density was evaluated in the intraepithelial and stromal compartment of the tumor. Results: The classification of tumors as “immune desert” when stromal CTL were ≤ 50 cells/mm2, “inflamed” when intraepithelial CTL were > 500 cells/mm2, and as “excluded” when neither of these definitions met these cut off values allowed the best discrimination regarding overall survival. These groups had median OS periods of 37, 61, and 85 months, respectively. In “immune desert” and “immune excluded” tumors high Treg tended to worsen OS, but in “inflamed” tumors high Treg clearly improved OS. Conclusions: We propose that, in locally advanced HNSCC, the tumor immune state “inflamed”, “immune excluded”, and “immune desert” can be defined by intraepithelial and stromal CTL. Tregs can further subdivide these groups. The opposing effects of Tregs in the different groups might be the reason for the inconsistency of Tregs prognostic values published earlier.
The current publication
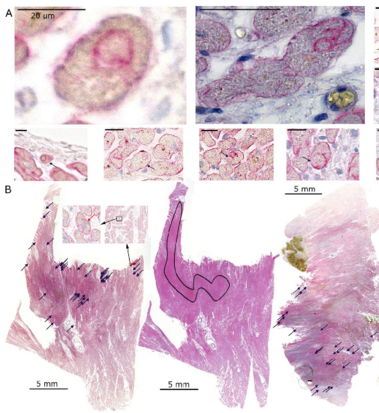
All normal cells can perform non-professional phagocytosis!
Non-professional phagocytosis: a general feature of normal tissue cells
Jacob C. Seeberg, Monika Loibl, Fabian Moser, Manuela Schwegler, Maike Büttner-Herold, Christoph Daniel, Felix B. Engel, Arndt Hartmann, Ursula Schlötzer-Schrehardt, Margarete Goppelt-Struebe, Vera Schellerer, Elisabeth Naschberger, Ingo Ganzleben, Lucie Heinzerling, Rainer Fietkau & Luitpold V. Distel.
Non-professional phagocytosis by cancer cells has been described for decades. Recently, non-professional phagocytosis by normal tissue cells has been reported, which prompted us to take a closer look at this phenomenon. Non-professional phagocytosis was studied by staining cultured cells with live-cell staining dyes or by staining paraffin-embedded tissues by immunohistochemistry. Here, we report that each of 21 normal tissue cell lines from seven different organs was capable of phagocytosis, including ex vivo cell cultures examined before the 3rd passage as well as the primary and virus-transformed cell lines. We extended our analysis to an in vivo setting, and we found the occurrence of non-professional phagocytosis in healthy skin biopsies immediately after resection. Using dystrophin immunohistochemistry for membrane staining, human post-infarction myocardial tissue was assessed. We found prominent signs of non-professional phagocytosis at the transition zone of healthy and infarcted myocardia. Taken together, our findings suggest that non-professional phagocytosis is a general feature of normal tissue cells.
Scientific Reports volume 9, Article number: 11875 (2019)