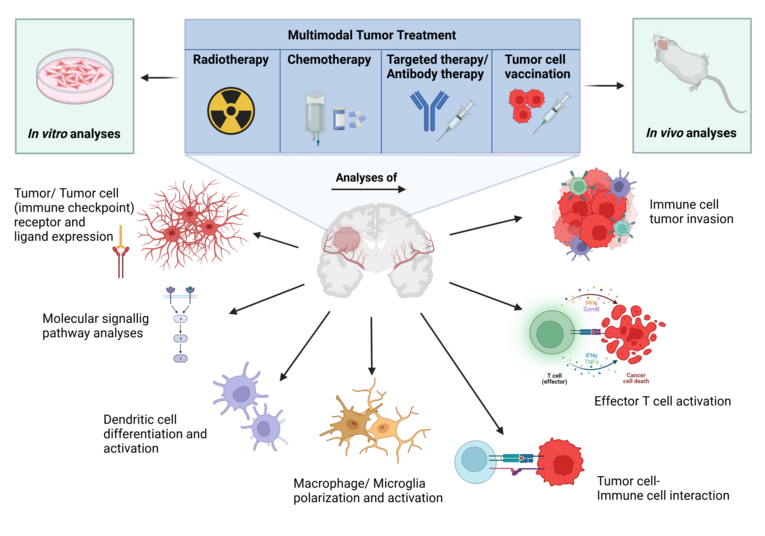
Immunobiology of brain tumors
Glioblastoma multiforme (GBM) is a primary high-grade astrocytoma (WHO grade IV) with a very poor prognosis for patients due to its brain localization, its infiltrative and fast growth and the suppression of a successful anti-tumor immune response. The current treatment includes resection as well as radio- and chemotherapy. Clinical Studies based on immunotherapy approaches lack the desired and expected results so far, which is why additional optimization of patient treatment is urgently needed. Our objective is to unravel potential cellular as well as molecular mechanisms underlying the tumor development and interaction with the immune system during therapy in order to gain improvement of therapeutic interventions for patients with malignant brain tumors.
The working group (funded by the DFG) has specialized in investigating immune therapeutic strategies, including whole-cell-vaccines, combining radiotherapy in pre-clinical model systems. Our research is based on in vitro and in vivo studies analyzing molecular signaling pathways, expression profiles and tumor/immune cell interaction as well as physiologically relevant GBM mouse models.
Therefore, various lab techniques are used, such as a detailed phenotypical assessment of tumor and immune cells via multicolor-flow cytometry, mRNA analysis via Real Time PCR and droplet digital PCR (ddPCR), protein detection via ELISA and Western Blot and cell analyses via fluorescence as well as transmitted light microscopy. The usage of specific pre-clinical, syngenic and orthotopic animal models can provide results that allow a more rapid realization in clinical studies.

Doctoral candicates - Medicine
Mona Shojaei
Celina Schuster
Theodhora Shuti
Selected Publications
I Research article
Normofractionated irradiation and not temozolomide modulates the immunogenic and oncogenic phenotype of human glioblastoma cell lines. Schatz J, Ladinig A, Fietkau R, Putz F, Gaipl US, Frey B, Derer A. Strahlenther Onkol. 2022 Dec 8. doi: 10.1007/s00066-022-02028-8.
Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Derer A*, Spiljar M*, Bäumler M, Hecht M, Fietkau R, Frey B, Gaipl US. Front Immunol.2016 Dec 22;7:610. doi: 10.3389/fimmu.2016.00610. *authors contributed equally
Rsk2 controls synovial fibroblast hyperplasia and the course of arthritis. Derer A*, Böhm C*, Grötsch B, Grün JR, Grützkau A, Stock M, Böhm S, Sehnert B, Gaipl U, Schett G, Hueber AJ, David JP. Ann Rheum Dis.2014 Nov 20. PMID:25414238 *authors contributed equally
Blockade of IL-36 receptor signaling does not prevent from TNF-induced arthritis. Derer A, Groetsch B, Harre U, Böhm C, Towne J, Schett G, Frey S, Hueber AJ. PLoS One. 2014 Aug 11;9(8):e101954. PMID: 25111378
RSK2 protects mice against TNF-induced bone loss. Böhm C*, Derer A*, Axmann R, Hillienhoff U, Zaiss MM, Luther J, Zech C, Stock M, Scholtysek C, Engelke K, Hess A, Tuckermann JP, Schett G, David JP. J Cell Sci.2012 May 1;125(Pt 9):2160-71. PMID: 22344264 *authors contributed equally
II Reviews
Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Deloch L*, Derer A*, Hartmann J, Frey B, Fietkau R, Gaipl US. Front Oncol. 2016 Jun 20;6:141. doi: 10.3389/fonc.2016.00141. Review. *authors contributed equally
Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Derer A, Frey B, Fietkau R, Gaipl US. Cancer Immunol Immunother. 2016 Jul;65(7):779-86. doi: 10.1007/s00262-015-1771-8. Review
Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses - pre-clinical evidence and ongoing clinical applications. Derer A*, Deloch L*, Rubner Y*, Fietkau R, Frey B, Gaipl US. Front Immunol. 2015 Oct 8;6:505. doi: 10.3389/fimmu.2015.00505. eCollection 2015. *authors contributed equally
Further publications of Dr. Anja Derer available on "Web of Science Researcher ID".
Project-related third party funding
DFG:
Impact and mechanisms of PD-L1, PD-L2 and EGF-R expression on glioma cells following radiochemotherapy and its consequences for combination with vaccination and PD-1 inhibition
Cooperation partners
Prof. Dr. Christoph Bert
Radiation Physics of the Radiation Oncology, Universitätsklinikum Erlangen
Prof. Dr.-Ing. Schlücker
Institute of Process Machinery and Systems Engineering, Friedrich Alexander Universität Erlangen-Nürnberg.
Dr. Bettina Grötsch
Department of Internal Medicine 3 and Institute of Clinical Immunology, Universitätsklinikum Erlangen
