Systemic Anti-Tumor Immune Responses
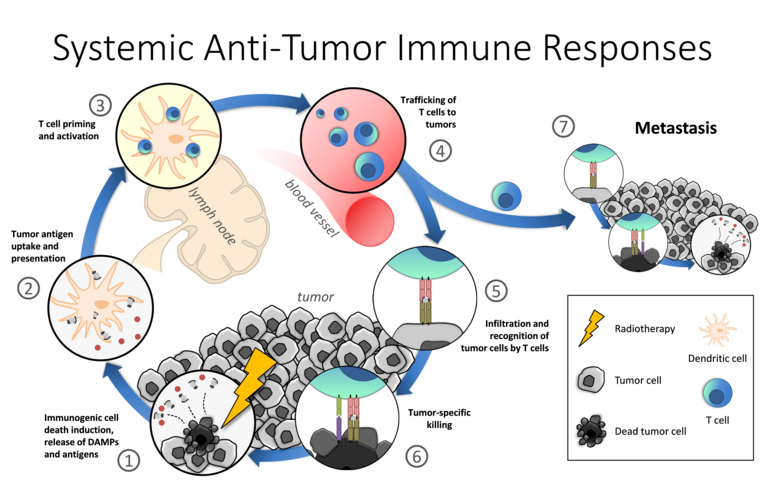
The primary goal of radiotherapy is to control the tumor locally by killing tumor cells with ionizing radiation. Modern radiation technology and planning make it possible to deposit a higher radiation dose per fraction in the tumor as part of hypofractionation protocols without affecting the surrounding normal tissue to a greater extent. As a result, the tumor cells increasingly die an immunogenic cell death and the microenvironment of the tumor is changed in such a way that an anti-tumor immune response is promoted. Ideally, this immune response is then not only directed against the locally irradiated tumor, but also has systemic effects on any metastases that may be present. However, the resulting reduction in tumor mass outside the irradiation field, also known as the "abscopal effect", has only rarely been observed in clinical practice to date.
Additional modulation of the immune system by immunotherapies, such as tumor vaccines, can enhance the anti-tumor immune responses triggered by radiotherapy or, in the case of immune checkpoint inhibitors, counteract the immunosuppressive microenvironment of the tumor. The combination of radiotherapy and immunotherapy has the potential to fundamentally improve cancer therapy in the future and to trigger systemic (abscopal) effects in patients more frequently. So far, however, little is known about how different radiotherapy parameters (dose, dose rate, fractionation, target volume, etc.) affect the immune system in different tumor entities, which immunotherapies are most suitable in each situation and how the individual therapies influence each other.
The aim of this working group is therefore to research the immunomodulatory effect of various combinations of radiation and immunotherapies using different preclinical models in order to better understand the immunological basis of systemic (abscopal) anti-tumor immune responses. This knowledge will then be used to design more effective clinical trials of radiation immunotherapies in the future.
Current projects include:
- The combination of hypofractionated radiation with a tumor cell vaccine generated under high pressure and immune checkpoint inhibitors. A particular focus is on tumor antigen presentation by dendritic cells. (IZKF junior project: J102: "cDC1s in abscopal effects and HHP vaccination" )
- The influence of less researched immune checkpoint molecules on immunosuppression by the tumor. (DFG Research Training Group GK 2599)
- The modulation of the complement system by ionizing radiation. (BMBF joint project ENDORSE [02NUK091B])
- The immune effects of cannabidiol (CBD) in combination with radiotherapy


Doctoral candidates - Medicine
Magnus Trottnow
Florian Schultz (GRK2599)

Methods
Multicolor Flow Cytometry
Preclinical Model Systems
CRISPR gene editing
high hydrostatic pressure inactivation of tumor cells
RNA sequencing
(Endpoint) PCR
Quantitative PCR
ELISA
Primary Cell Cultures
MSD System (Multi-Array Technology)
Selected Publications
Gehre, S., Meyer, F., Sengedorj, A., Grottker, F., Reichardt, C. M., Alomo, J., Borgmann, K., Frey, B., Fietkau, R., Rückert, M., & Gaipl, U. S. (2023). Clonogenicity-based radioresistance determines the expression of immune suppressive immune checkpoint molecules after hypofractionated irradiation of MDA-MB-231 triple-negative breast cancer cells. Frontiers in oncology, 13, 981239. https://doi.org/10.3389/fonc.2023.981239
Wedekind H, Walz K, Buchbender M, Rieckmann T, Strasser E, Grottker F, Fietkau R, Frey B, Gaipl US, Rückert M. Head and neck tumor cells treated with hypofractionated irradiation die via apoptosis and are better taken up by M1-like macrophages. Strahlenther Onkol. 2022 Feb;198(2):171-182. doi.org/10.1007/s00066-021-01856-4.
Rückert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the immune system: More than just immune suppression. Stem Cells. 2021 Sep;39(9):1155-1165. doi.org/10.1002/stem.3391
Rückert M, Deloch L, Frey B, Schlücker E, Fietkau R, Gaipl US. Combinations of Radiotherapy with Vaccination and Immune Checkpoint Inhibition Differently Affect Primary and Abscopal Tumor Growth and the Tumor Microenvironment. Cancers (Basel). 2021 Feb 9;13(4):714.
doi.org/10.3390/cancers13040714
Seitz C, Rückert M, Deloch L, Weiss EM, Utz S, Izydor M, Ebel N, Schlücker E, Fietkau R, Gaipl US, Frey B. Tumor Cell-Based Vaccine Generated With High Hydrostatic Pressure Synergizes With Radiotherapy by Generating a Favorable Anti-tumor Immune Microenvironment.Front Oncol. 2019 Aug 28;9:805.
doi.org/10.3389/fonc.2019.00805
Further publications of Dr. Michael Rückert available on "Web of Science Researcher ID".
Project-related third party funding
BMBF Verbundprojekt ENDORSE [02NUK091B]:
Einfluss von(dicht-)ionisierender Strahlung auf die immunmodulatorische Wirkung des Komplementsystems: Neue Perspektiven für Gesundheitsschutz und Tumortherapie
Stellvertretender Verbundkoordinator und Projektleiter
IZKF junior project leader: J102 “cDC1s in abscopal effects and HHP vaccination”
Cooperations
Prof. Dr. med. Dr. h.c. Friedrich Paulsen
Institut für Funktionelle und Klinische Anatomie, Friedrich-Alexander-Universität Erlangen-Nürnberg
Prof. Dr.-Ing. Schlücker
Lehrstuhl für Prozessmaschinen und Anlagentechnik, Friedrich-Alexander-Universität Erlangen-Nürnberg
Prof. Dr.-Ing. Michael Wensing
Department Chemie- und Bioingenieurwesen (CBI), Friedrich-Alexander-Universität Erlangen-Nürnberg
Prof. Dr. Claudia Fournier und Dr. Alexander Helm
GSI Helmholtzzentrum für Schwerionenforschung GmbH, Darmstadt
Fulvia Vascotto
TRON - Translationale Onkologie an der Universitätsmedizin der Johannes Gutenberg Universität Mainz gemeinnützige GmbH
Prof. Dr. Alberto Mantovani, Prof. Dr. Cecilia Garlanda, Dr. Elena Magrini
Humanitas Universität, Mailand, Italien
PD Dr. Franziska Eckert
Universitätsklinik für Radioonkologie, Medizinische Universität Wien, Österreich
